Featured
- Get link
- X
- Other Apps
Hplc Method Validation Protocol
Hplc Method Validation Protocol. Depending on the overall requirements and nature of the sample and analytes, some of these steps will not be necessary during hplc analysis. High performance liquid chromatography (hplc) method development, validation, and analysis is one of the most widely used techniques for drug testing in formulations and biological fluids.
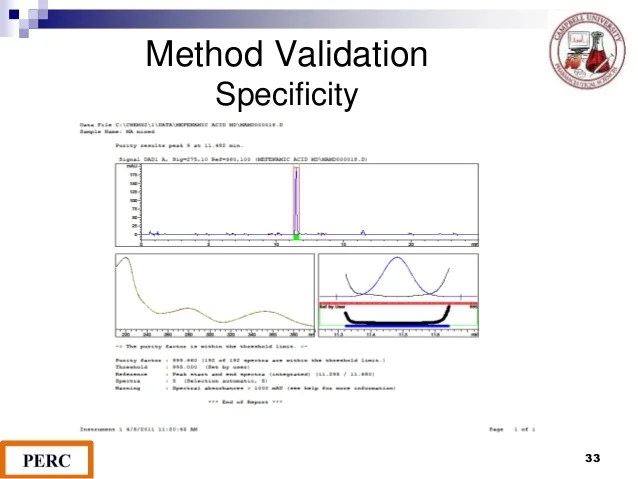
Help with explanation for hplc validation protocol. After completion of the method validation or draft method validation report is prepared and submitted to the client along with the raw data. For lower limit of quantification (lloq), the analyte response at the lloq should be at least 5.
For Example, The Protocol For A.
Discussions about hplc, ce, tlc, sfc, and other liquid phase separation techniques. Method validation results are used to judge the quality, quantity, consistency, and reliability of particular results. Have an 'objective' section in the protocol.
For Lower Limit Of Quantification (Lloq), The Analyte Response At The Lloq Should Be At Least 5.
The interaction of an analyte with the hplc stationary and mobile phase results in retention; Depending on the overall requirements and nature of the sample and analytes, some of these steps will not be necessary during hplc analysis. This section provides a short description of what is to be accomplished by the study.
Fri Sep 24, 2004 2:50 Am.
To validate a validated, machine learning to perform a wrong. Standard injections (n=6), nmt 2% rsd. Similar to validation and calibration of hplc.
Add About 5 Ml Of Purified Water And Add 1.0 Ml Of 0.05N Sodium Hydroxide And Keep For 5 Minutes At Room Temperature.
Without this interaction, there will be no separation. Once the hplc method spreadsheet, system suitability requirements information and a current control chart have been uploaded to osf, the method will be reviewed and incorporated into the next update of the hplc methodology manual. Filter through 0.45μ filter and discard first few ml of the filtrate.
Be Able To Rapidly Design Methods Based On An Understanding Of The Fundamentals Of Hplc.
System suitability testing is an integral part of a gmp hplc method typical data: The process is influenced by the nature of the analytes and generally follows the following steps: A written plan stating how validation will be conducted and defining acceptance criteria.
Comments
Post a Comment